
The ideal joint replacement for CMC thumb arthritis.
Revolutionary treatment option for a thumb CMC joint damaged by osteoarthritis.
Developed by renowned U.S. hand surgeon Nathan T. Morrell along with an expert science and engineering team, the ZEPART® implant is currently in the testing phase with anticipated launch in 2026.
The ZEPART® Implant

Engineered to adapt to the hand’s natural movements.
Without a suitable joint replacement, surgeons are reduced to joint removal or limited workarounds for a painful thumb CMC joint damaged by osteoarthritis. Our revolutionary implant meets the surgeon’s need for a stable, functional, adaptable thumb CMC joint replacement. Implementation of the ZEPART joint replacement has the potential to address pain management and restore function for the 200,000+ patients annually who seek a surgical intervention in the U.S. alone.
The device shown is currently under development and has not yet received FDA clearance or approval. It is not available for sale in the United States.
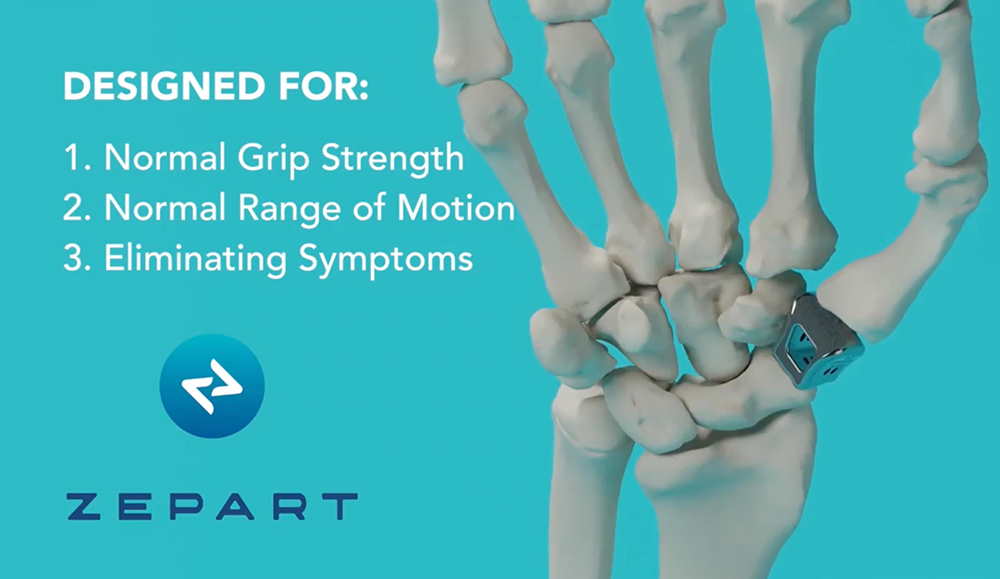
How it works.
ZEPART is an innovative thumb joint implant for carpometacarpal osteoarthritis surgery, designed to eliminate pain, restore mobility and improve quality of life. The implant is a stemmed device used for complete replacement of the human trapezium bone. It replicates the hand’s natural geometry to articulate normally with neighboring carpal bones – no resurfacing or ball-and-sockets required. The implant is designed to prevent thumb shortening and maintain metacarpal alignment and natural resting position of the thumb. With ZEPART, natural movement is restored.
Developed by Sandia Medical Technologies in Albuquerque, New Mexico.
Our all-star team is the emerging R&D leader for orthopedic surgery and related fields.
